Ion Chromatography
Posted on October 3, 2023 Analytical Chemistry
This post was originally published in three parts which have been combined below.
Part 1
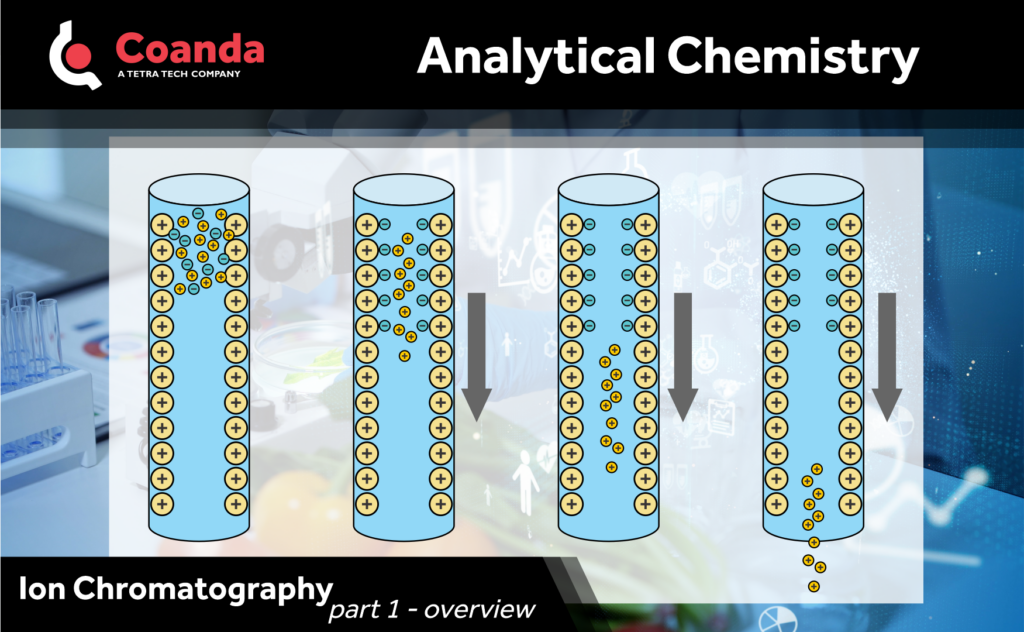
Ion chromatography (IC) is a powerful analytical technique used for the separation, identification, and quantification of ions in various samples. It has gained popularity across scientific disciplines due to its versatility, sensitivity, and broad range of applications. In this post, we will delve into the principles and instrumentation, and will cover applications, and advantages in future posts.
IC is based on the principles of liquid chromatography (LC), where the separation of ions occurs using a stationary phase and a mobile phase. The stationary phase in ion chromatography consists of an ion exchange resin, which selectively interacts with ions in the sample based on their charge and size. The mobile phase is typically an aqueous solution containing various salts or buffers that help elute the ions of interest.
The separation process in IC relies on the competition between the sample ions and the ions present in the mobile phase for binding sites on the stationary phase. By adjusting the composition and concentration of the mobile phase, different ions can be eluted at specific retention times, allowing for their separation.
Instrumentation:
IC systems consist of several key components that work together to achieve efficient separation and detection of ions. These components include:
- Pump: The pump delivers the mobile phase at a constant flow rate to ensure reproducibility and stability of the separation.
- Sample Injector: The sample injector introduces the sample into the mobile phase, either through an automatic injector or a manual injection loop.
- Column: The column contains the stationary phase, which is responsible for the separation of ions. Ion exchange columns are commonly used, with variations such as anion exchange columns and cation exchange columns.
- Detector: The detector detects and quantifies the separated ions. The most commonly used detectors in IC include conductivity detectors, ultraviolet (UV) detectors, and mass spectrometers.
Part 2
In our previous post, we outlined the principles of Ion Chromatography (IC) and instrumentation, today we cover IC applications, and will discuss its advantages in a future post.
Applications:
IC finds applications in various scientific fields due to its ability to separate and quantify a wide range of ions. Some key application areas include:
- Environmental Analysis: IC plays a crucial role in the analysis of environmental samples, such as water and soil. It enables the determination of various inorganic and organic ions, including common pollutants such as nitrates, phosphates, sulfates, and heavy metals. By monitoring these ions, environmental scientists can assess the quality of water sources and the impact of human activities on the environment.
- Pharmaceutical Analysis: In pharmaceutical research and quality control, IC is used for the analysis of pharmaceutical ingredients, excipients, and degradation products. It enables the detection and quantification of ions like chloride, sulfate, and phosphate, which are critical for ensuring the safety and efficacy of pharmaceutical products.
- Food and Beverage Analysis: IC is widely employed in the analysis of food and beverage samples to determine the levels of various ions, including anions (e.g., chloride, nitrate, and sulfate) and cations (e.g., sodium, potassium, and calcium). This information helps ensure food safety, assess nutritional content, and monitor the quality and authenticity of food and beverage products.
- Water Quality Analysis: IC plays a pivotal role in water quality analysis, allowing the determination of anions and cations that influence taste, corrosion, and scaling. By measuring ions such as fluoride, chloride, nitrate, sulfate, and carbonate, water treatment facilities can optimize their processes to provide safe and desirable drinking water to the public.
Part 3

Earlier posts described the principles of Ion Chromatography (IC), relevant instrumentation, and common IC applications. Today’s post concludes the series by exploring its advantages.
Advantages of IC
- High Selectivity: IC provides excellent selectivity for different ions based on their charge, size, and functional groups. This allows for the separation and analysis of complex mixtures with high precision and accuracy.
- Sensitivity: IC is capable of detecting ions at trace levels, often in the parts-per-billion (ppb) or even parts-per-trillion (ppt) range. This high sensitivity enables the analysis of samples with low ion concentrations, ensuring accurate results.
- Versatility: IC can analyze a wide range of sample types, including aqueous samples, organic solvents, and complex matrices. It can also handle a diverse range of analytes, including inorganic ions, organic acids, and polar compounds.
- Automation and Throughput: With modern advancements in instrumentation and automation, ion chromatography systems offer high sample throughput, reducing analysis time and increasing efficiency in laboratories.
- Ion chromatography is a powerful analytical technique that allows for the separation, identification, and quantification of ions in various samples. With its selectivity, sensitivity, and broad range of applications, IC has become an indispensable tool in numerous scientific disciplines. Its ability to analyze complex mixtures, detect trace levels of ions, and provide accurate results makes it a valuable asset in environmental analysis, pharmaceutical research, food and beverage analysis, and water quality assessment.


