Oil Upgrading & Refining
Posted on March 28, 2023 Oil & Gas Process Engineering
This post was originally published in three parts which have been combined below.
Part 1
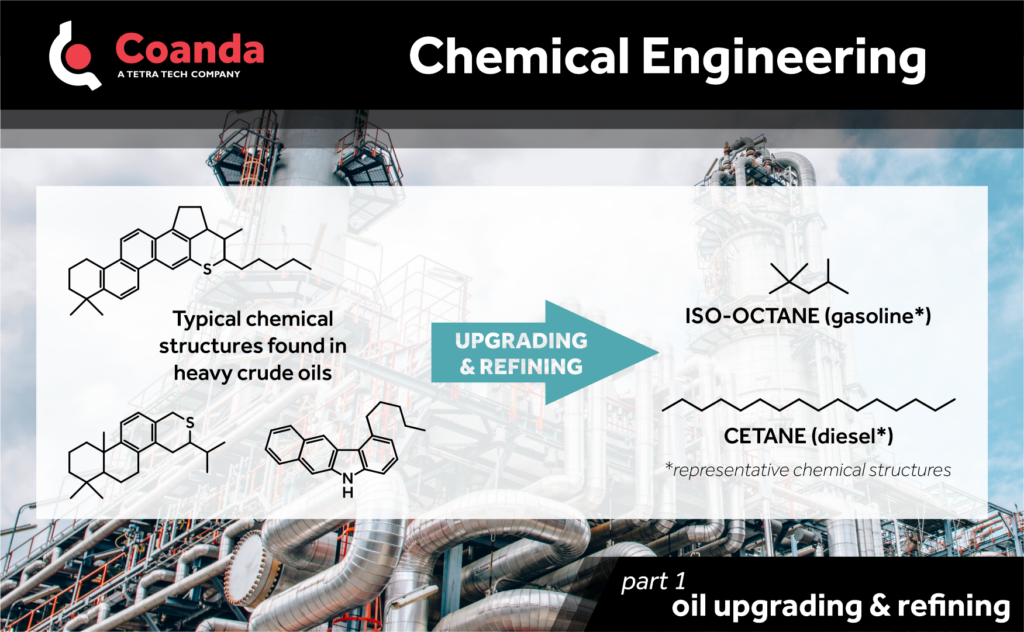
Oil upgrading and refining facilities process petroleum feedstocks and convert them into a variety of petroleum products, with an emphasis on the production of liquid fuels such as gasoline, diesel, and jet fuel. A suite of chemical processes is deployed in these facilities to address 3 main chemistry differences between the petroleum feedstocks and the targeted products:
1. Hydrocarbon molecules in useful fuel products are smaller than most found in petroleum feedstocks. For example, gasoline compounds have mostly 6-10 carbon atoms, whereas crude oil compounds would have an average molecular size around 20-35 carbon atoms. The conversion of heavy petroleum fractions into lighter products is achieved by cracking processes.
2. Petroleum feedstocks usually have a lower hydrogen-to-carbon ratios than the hydrocarbons making up useful fuel products. Two pathways are available to modify the hydrogen-to-carbon ratios of petroleum material: hydrogen-addition processes and carbon-rejection processes.
3. Petroleum feedstocks usually comprise of significant quantities of heteroatoms (namely nitrogen, sulphur, and metals). For example, bitumen from the Canadian oil sands has typically more than 4.5 wt.% sulphur and 0.5 wt.% nitrogen. These elements are undesirable in fuel products, since their combustion would generate NOx and SOx, which contribute to air pollution and the formation of acid rain; and metals affect subsequent processes and can cause deposits in combustion. Over the past decades, continuously tighter specifications on the sulphur content in fuels have been imposed by regulators from most countries to mitigate this environmental impact. Processes involving catalytic reactions with hydrogen are usually employed to remove these heteroatoms.
The next two posts will describe these chemical processes in further detail.
Part 2

Hydroprocessing is a group of chemical processes that involve the addition of hydrogen to hydrocarbon mixtures in oil upgrading and refining facilities to transform heavy hydrocarbon feedstocks to final usable fuel products.
Those processes include hydroconversion, hydrotreatment, and hydrocracking; each one of them having a different goal. Although the exact definition of these processes may be a bit subjective, however, they can be broadly described as follows:
-Hydroconversion is used in primary upgrading to remove the bulk of the heteroatoms (i.e., any atom other than carbon or hydrogen) and to partially break down large molecules into smaller, easier to process molecules.
Hydrotreatment and hydrocracking are used in secondary upgrading (or refining) to yield the finished products:
-Hydrotreatment is used to reduce the number of heteroatoms and saturate the hydrocarbons without significant thermal breakdown.
-Hydrocracking uses bi-functional catalysts to promote the breakdown of feedstock into lighter hydrocarbons but the catalytic process is sensitive to the presence of heteroatoms in the feed material.
Nowadays, the use of hydroprocessing is not limited to conventional oil upgrading; but it has also been proposed for bio-oil and pyrolysis oil upgrading. Typically, bio-oils have an oxygen content between 30 to 50%. The presence of oxygen in these feedstocks can cause several problems including corrosion, polymerization, and low heating value in the finished products.
Depending on the source, pyrolysis oil can contain other impurities including nitrogen, chloride, and metals. While chloride cannot be removed with hydroprocessing and requires additional treatment, nitrogen, oxygen, and metals can be removed by such processes.
Hydroprocessing of pyrolysis oils has been proposed as a route to produce intermediate petrochemical products and drop-in fuels which has opened another way to recycle different types of waste into useful products.
Part 3

We previously introduced one of the routes to increase the hydrogen-to-carbon ratio (H/C) in a petroleum feedstock using carbon-rejection methods. As the name implies, these processes rely on the formation of carbon-rich solids, known as coke, which are removed from the hydrocarbon product stream.
In coking operations, the feedstock is heated to high temperatures (> 485 °C) to cause severe thermal cracking, which is an endothermic process. Smaller, more aliphatic products are cleaved from the large hydrocarbon molecules, while other molecular fragments condense and form coke. The resulting solids have an atomic H/C ratio below 1 and tend to concentrate heteroatoms (aromatic sulphur and nitrogen, metals). With the rejection of coke, this process achieves the 3 main chemical transformations between feedstock and products mentioned previously: increasing the H/C ratio, reducing molecular sizes and removing heteroatoms.
Two main techniques have been developed for commercial coking operations: delayed coking and fluid coking. Delayed coking is a semi-batch process where the coke accumulates inside the reactor, which is then taken offline to remove the coke. In fluid coking, which operates continuously, the produced coke particles are partially burned before being reintroduced into the reactor to supply heat for the reaction.
While the rejection of carbon reduces the yield of usable products, coking processes are less expensive in terms of capital and operation costs compared to hydrogen addition processes. Selection between carbon rejection and hydrogen addition processes depends mostly on market conditions and price forecasting.


