Vapour-Liquid Equilibria (VLE)
Posted on October 27, 2022 Analytical Chemistry
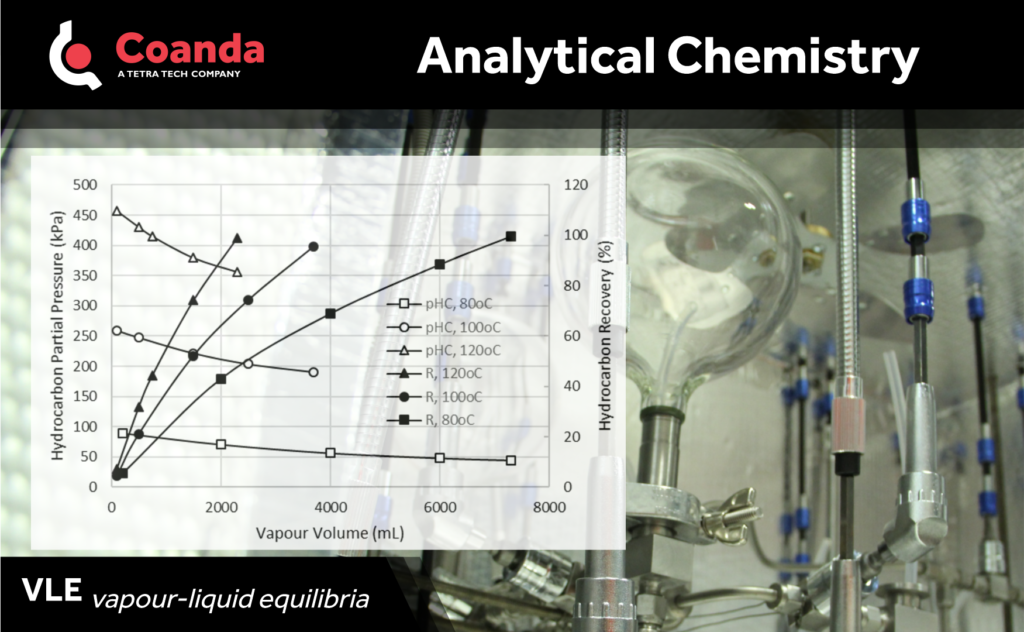
Experimental determination of Vapour-Liquid Equilibria (VLE) is an essential element for designing and operating separation processes based on differential volatility. This is particularly true for complex sample matrices where a model for the phase behaviour is a priori unknown. Working with Dr D.Y. Peng, who pioneered the technique, Coanda designed and built an experimental apparatus for partitioning samples into their vapour and condensed phases under a set temperature and total volume conditions, which constrains equilibrium pressure.
In a VLE experiment, samples are introduced in their condensed phase inside a cell which has been purged of its headspace. The sample cell is initially isolated from the glass bulb assembly, which is going to be the receptable of the sample vapours.
Both the sample cell and the glass bulb assembly are placed inside a tightly-temperature-controlled convection oven with a maximum operating temperature of 120°C.
At the beginning of the experiment, the glass bulb assembly is evacuated while the oven temperature is reaching the desired setpoint. Once the oven temperature is stable at its setpoint and the glass bulb assembly has no residual pressure, a valve is opened to allow for the sample vapours to expand into the glass bulb assembly.
A solenoid pump recirculates the vapours through the unit and back into the sample cell to facilitate equilibration. Equilibration is deemed completed once the system pressure becomes stable within ±0.001psi. The glass bulbs and the sample cells can be each isolated for the individual characterization of the vapours and the residual condensed phase obtained at equilibrium conditions.