Direct Air Capture (DAC)
Posted on March 14, 2024 Cleantech
Previously, we discussed strategies for achieving a carbon neutral economy. However, while achieving net-zero emissions will stop atmospheric CO2 concentrations from further increasing, reversing effects of greenhouse gas emissions can only be achieved by removing carbon-dioxide already in the atmosphere. Thus, lowering levels of atmospheric CO2 requires us to not only treat sources of carbon emissions, but also the atmosphere itself. As a result, direct air capture (DAC) of CO2 has seen a surge in interest and investment over the past few years, with a growing number of companies entering the space.
One of the main advantages of DAC is that, because atmospheric CO2 concentration is fairly uniform, capture efficiency is not highly location dependent. In other words, DAC can be deployed wherever convenient, such as on marginal land or adjacent to natural storage sites.
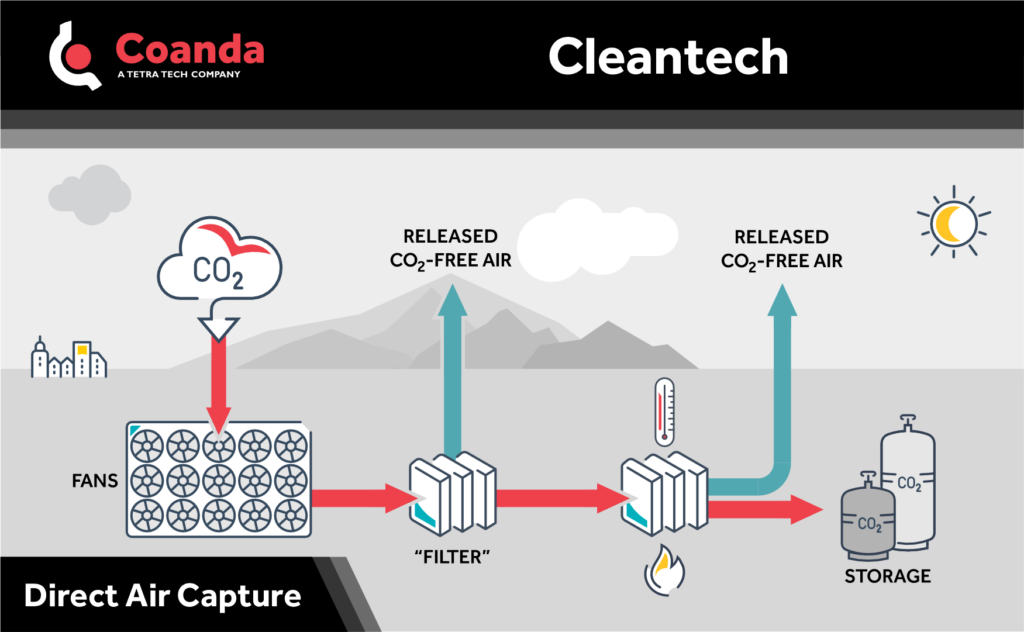
The primary challenge involved with direct air capture of CO2, however, is due to the relatively low concentration of CO2 in air. While CO2 concentrations in flue gas can sometimes be upwards of 20%, atmospheric concentration currently averages about 0.04%. This means that to achieve the same gross CO2 capture, thousands of times more air must be processed, and with a much weaker concentration gradient driving the process. To have a significant impact, technologies used for direct air capture must therefore be more efficient and deployed at a larger scale than those typically used for point-source capture.
There are generally two approaches to direct air capture: air is either bubbled through a solvent liquid or flows in contact with a solid sorbent, to which it bonds. In both cases the solvent or sorbent is heated to release the CO2. The heat of regeneration and storage capacity of a material are among the most important factors for determining the viability of a technology, and developing new or improved materials and processes is the topic of intense research effort.


