Geologic CO2 Sequestration
Posted on October 4, 2024 Cleantech
In a previous post, we introduced the topic of sequestration and storage, providing examples of natural storage sites. However, the capacity of the oceans, plant life, and natural rock formations for permanent storage within a timeframe needed to lessen the impact of climate change is limited. We must therefore look for other safe and permanent storage sites for carbon removed from the atmosphere. In this post, we introduce proposed methods of trapping captured CO2 underground in geological formations.
Geologic sequestration refers to the long-term storage of gaseous, liquid, or supercritical carbon dioxide in underground sites. The current focus is primarily on storage in saline formations, depleted oil and gas reservoirs, and unmineable coal seams. However, before any solution(s) can be implemented, it is first necessary to understand how CO2 will behave once injected underground, in order to minimize leakage back to the surface.
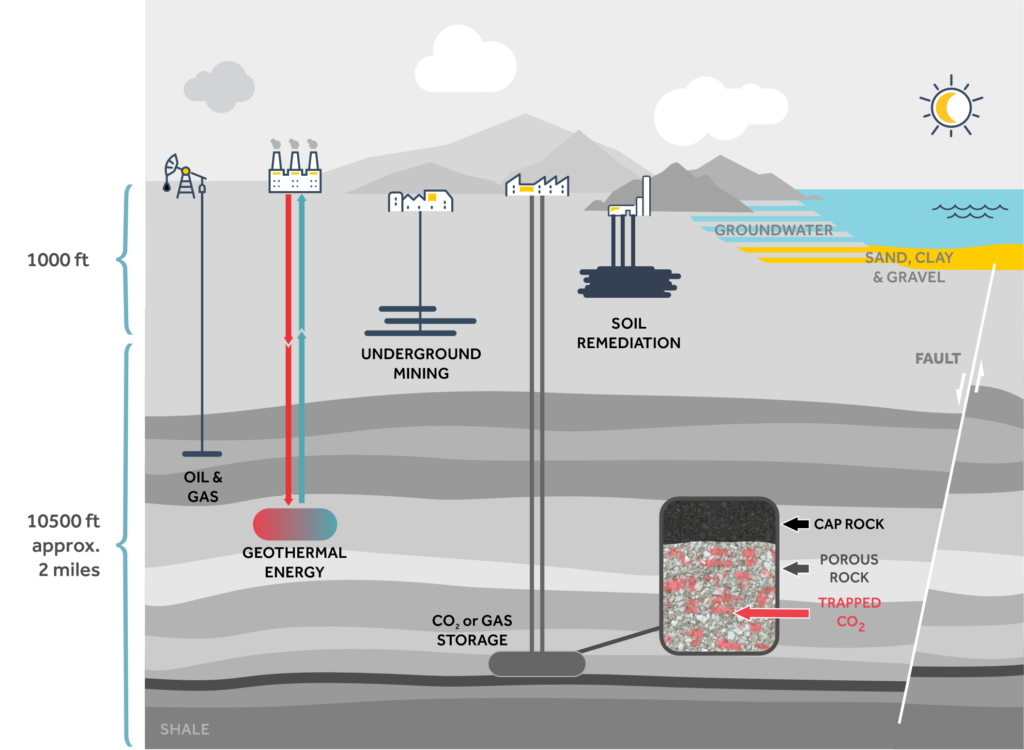
Geologic Carbon Sequestration
Storing CO2 in a gaseous state is in many ways the easiest solution, as it requires the least power to compress the gas and inject it into a reservoir. However, it is also the least stable solution. Because of its low pressure (compared with liquid or supercritical CO2), such sites would be located nearest to the surface, at depths less than about 300 m, and because of its low viscosity, any leakage would flow rapidly, returning CO2 directly back into the atmosphere.
At depths between approximately 300 m and 600 m, the conditions of pressure and temperature favour storage of CO2 in its liquid state. This is considered to be a more stable long-term solution, as the fluid properties and greater depth both work to prevent leakage to the surface and back into the atmosphere.
Beyond about 600 m depth, the conditions of temperature and pressure correspond to those of CO2 in its supercritical state. This range of depth includes a large number of depleted wells and natural gas reservoirs, unmineable coal seams, and other potential storage sites. CO2 has a critical point of 31.1°C and 7.38 MPa. At temperatures and pressures at or above the critical point, there are no longer distinct liquid and gaseous phases, and the fluid has behaviours of both. Supercritical CO2 (sCO2) is often used industrially and has well understood properties. However, its properties and behaviours in geologic formations are not yet sufficiently well understood, and this remains an area of significant research effort.


