Contact Angle
Posted on January 2, 2025 Physical Modeling
The angle formed between the interfaces of three phases – typically, a solid, liquid, and gas – is referred to as the contact angle. Very often, the liquid of interest is water, and the contact angle is conventionally measured through the water phase from the solid-liquid interface to the gas-liquid interface. The contact angle of a water drop on a piece of solid material in air is very often used to characterize how hydrophobic or hydrophilic that material is.
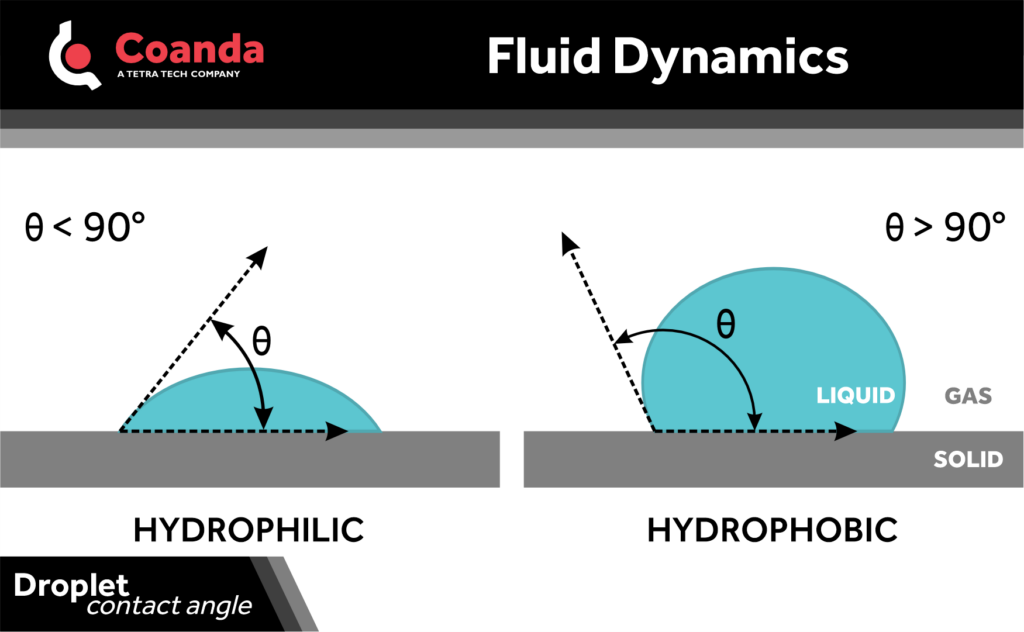
Water beads up on hydrophobic materials, which is good where water-repellent surfaces are desired. This corresponds to a high contact angle. On hydrophilic materials, water tends to spread out, having a lower contact angle. This can be useful, for example, in evaporative cooling where a large contact area between water droplets and a surface is helpful. Hydrophilicity is also good when a material needs to maintain a layer of water at its surface.
At its simplest, the contact angle results from the balance of interfacial tensions at the contact line where three phases of matter meet. Interfacial tension is the force that occurs along the interface between different materials due to the discontinuity in phases and cohesive forces between molecules. It is most familiar as the surface tension of water that, absent other significant forces, pulls water droplets or bubbles in water into a spherical shape.
The interfacial tension is a measure of how thermodynamically favorable it is to produce an interface. Nature seeks to minimize the energy in a system, therefore the contact angle of a drop of liquid on a surface reflects the configuration that is most thermodynamically favorable to achieve this. This means that, on a hydrophilic surface, energy is minimized by the drop spreading, whereas on a hydrophobic surface, the area covered by the drop is reduced by the higher contact angle. That’s why surfaces like very clean glass are hydrophilic, whereas Teflon is hydrophobic – the surface energies cause different spreading behavior.
Contact angles play a role in other systems as well. For example, the contact angle of a mineral particle in a flotation process determines how easily that type of particle can be recovered. Hydrophobic particles are more easily recovered than hydrophilic particles.
Chemical treatments are applied in some processes to make particles more hydrophobic and thus increase recovery. Like water drop contact angles, particle contact angles with bubbles or drops can also be measured using a variety of methods, and can be used to understand particle behavior in processes and tailor behavior in slurry and other multiphase systems.


